Suat Zengin, Behcet A, Sahin Karta, Basri Can, Mustafa Orkmez, Abdullah Taskın, Ugur Lok, Bediha Gulen, Cuma Yildirim, Seyithan Taysi
1Department of Emergency Medicine, Gaziantep University School of Medicine , Gaziantep, Turkey
2Department of Emergency Medicine, Şehitkamil State Hospital, Gaziantep, Turkey
3Medical Biochemistry, Gaziantep University School of Medicine , Gaziantep, Turkey
4Department of Biochemistry, Harran University School of Medicine, Sanliurfa, Turkey
5Department of Emergency Medicine, Adıyaman University, Adıyaman, Turkey
6Department of Emergency Medicine, Bezmialem Vakıf University, Istanbul, Turkey
Corresponding Author:Suat Zengin, Email: zengins76@gmail.com
An assessment of antioxidant status in patients with carbon monoxide poisoning
Suat Zengin1, Behcet A1, Sahin Karta2, Basri Can1, Mustafa Orkmez3, Abdullah Taskın4, Ugur Lok5, Bediha Gulen6, Cuma Yildirim1, Seyithan Taysi3
1Department of Emergency Medicine, Gaziantep University School of Medicine , Gaziantep, Turkey
2Department of Emergency Medicine, Şehitkamil State Hospital, Gaziantep, Turkey
3Medical Biochemistry, Gaziantep University School of Medicine , Gaziantep, Turkey
4Department of Biochemistry, Harran University School of Medicine, Sanliurfa, Turkey
5Department of Emergency Medicine, Adıyaman University, Adıyaman, Turkey
6Department of Emergency Medicine, Bezmialem Vakıf University, Istanbul, Turkey
Corresponding Author:Suat Zengin, Email: zengins76@gmail.com
BACKGROUND:Carbon monoxide poisoning (COP) is an important cause of mortality and morbidity worldwide. This study was to investigate the levels of serum paraoxonase (PON), arylesterase (ARYL), ceruloplasmin (Cp), and sulfhydryl (-SH) in the treatment of COP, and to further understand the pathophysiology of COP.
METHODS:This prospective study comprised 107 individuals with COP (group 1) and 50 healthy volunteers (group 2). Serum, plasma, and erythrocyte samples were taken on admission from all participants with COP. This process was repeated in the 90thand 180thminutes of treatment. Samples were taken from the control group only once. The levels of plasma PON, ARYL, Cp activity and -SH were measured in both groups.
RESULTS:Age, gender, and carboxyhemoglobin level were not correlated with PON, ARYL, Cp, and -SH levels. PON, ARYL, and -SH levels were signi fi cantly decreased in group 1 compared with group 2. Conversely, Cp was signi fi cantly elevated in group 1 in contrast to group 2. Although ARYL was lower on admission in patients with COP than that was observed in the 90thand 180thminutes (P<0.001), Cp was higher on admission than at the other time points (P<0.001).
CONCLUSIONS:Participants with COP had decreased levels of antioxidants (PON, ARLY, and -SH). COP represses the antioxidant system.
Carbon monoxide poisoning; Paraoxonase; Arylesterase; Ceruloplasmin; Total sulfhydryl groups
INTRODUCTION
Carbon monoxide (CO) is a colorless, odorless, tasteless, and nonirritant gas that is primarily produced as a result of the incomplete combustion of any carbonaceous fossil fuel.[1,2]CO poisoning (COP) is a serious health problem worldwide. Exposure to high concentrations is lethal, resulting in approximately 50 000 visits to the emergency department and 2 700 deaths annually in the United States.[1–3]The non-speci fi c symptoms of CO exposure include headache, nausea, vomiting, palpitations, dizziness, and confusion. As exposure increases, patients develop more pronounced and severe symptoms, and oxygen-dependent organs, such as the brain and heart, show the earliest signs of injury.[1,2]The diagnosis of COP depends primarily on the history of exposure, but an exact diagnosis is possiblethrough a measurement of high CO hemoglobin (COHb) levels.[1,2,4]The standard treatment is the administration of 100% oxygen, which should be continued until the COHb level returns to normal.[1,2]
Free radicals are defined as molecules or molecular fragments that contain one or more unpaired electrons in atomic or molecular orbitals.[5]Although they have a very short life cycle, free radicals are very harmful to organisms because of their activity.[5]To prevent free radical damage, the body normally uses its antioxidant defense system, but if antioxidants are unavailable or if free-radical production becomes excessive, tissue damage can occur. Free radicals interact with biomolecules such as carbohydrates, lipids, proteins, and DNA, thus leading to structural and metabolic alterations in cells. This results in tissue damage in vital organs, including the heart, kidneys, liver, stomach, lungs, and brain.[5]
Paraoxonase-1 (PON1) is a high-density lipoprotein (HDL)-associated enzyme with three activities: paraoxonase (PON), arylesterase (ARYL), and dyazoxonase.[6–8]PON protects low-density lipoprotein (LDL) and HDL from oxidation.[9–11]Human serum PON and ARYL are enzymes of the esterase group that are encoded by the same gene, and have similar active centers.[12]ARYL is recognized as an antioxidant enzyme because it hydrolyzes lipid peroxides and oxidizes lipoproteins as paraoxonase.[7,13]PON and ARYL have been reported to decrease in many patients with hypercholesterolemia, diabetes mellitus, cardiovascular disease, liver and kidney diseases, and iron deviancy anemia.[7,11,14]Ceruloplasmin (Cp) which contains copper atoms is a plasma protein.[15]Cp permits the incorporation of iron into transferrin without the formation of toxic iron products. Under physiologic conditions, Cp is also important in the control of membrane lipid oxidation, likely by direct oxidation of cations, thus preventing their catalysis of lipid peroxidation.[16]Another antioxidant group is the total sulfhydryl (-SH) group, which plays a crucial role in protecting cells from oxidative damage by interacting with the electrophilic group of reactive oxygen species (ROS) as the first and major member of the physiologic antioxidant defense system.[17]In humans, decreased levels of -SH have been shown to cause various disorders, such as liver failure, coronary artery disease, stroke, and other neurological disorders.[17,18]
The present study aimed to investigate serum PON, ARYL, Cp and -SH levels in the treatment of COP, and to increase our understanding about the pathophysiology of COP.
METHODS
This study was conducted at the Department of Emergency and Clinical Biochemistry of Gaziantep University, Turkey from 2011 to 2012. The study protocol conforms to the principles of the Helsinki Declaration, and was approved by the Medical Ethics Committee of Gaziantep University. All participants gave written informed consent.
This study comprised 157 individuals, of whom 107 were enrolled in a COP group (group 1) and the remaining 50 in a control group (group 2). The control group consisted of healthy individuals who were administrative staff of our hospital (Table 1).
All participants were free of acute or chronic diseases, and were of normal body habitus. In addition, they submitted a detailed medical history and underwent a physical examination by physicians. Participants who suffered from coronary artery disease, hypercholesterolemia, hypertension, neurological disorders, diabetes mellitus, liver and kidney diseases, peripheral vascular disease, lung disease, multiple sclerosis, iron deficiency, or anemia, as well as those with obesity seen after submission of the medical history, physical examination, and laboratory examination were excluded.
Body mass index, blood sample collection and COHb level measurements
Body mass index was calculated by dividing weight in kilograms by height in meters squared (kg/m2). Blood samples were withdrawn from a cubital vein into blood tubes, and immediately stored on ice at 4 °C. Then, serum was separated from cells by centrifugation at 3 000 r/min for 10 minutes. The serum samples were stored at –80 °C until analysis. Blood samples for antioxidant study were taken once from group 2 and three times from group 1: on admission, and at the 90thand 180thminutes of treatment. COHb level measurements were performed using a signal extraction pulse CO-oximetry device (Masimo SET Rainbow, Masimo Co., USA). All theparticipants in group 1 were given 100% oxygen using a non-breather face mask, and none of them received hyberbaric oxygen therapy.
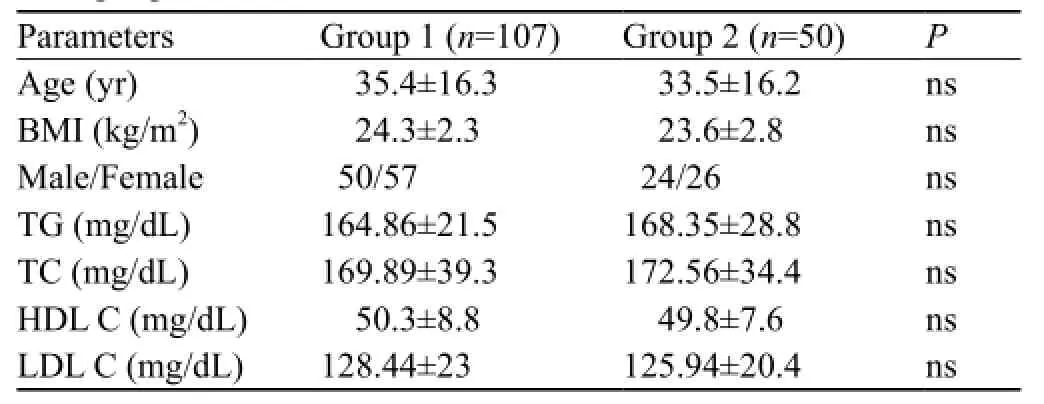
Table 1. Comparison of groups 1 and 2 in terms of clinical characteristics and lipid pro fi les
Measurement of paraoxonase and arylesterase activity
PON was measured in basal activity. The rate of paraoxon hydrolysis (diethyl-p-nitrophenylphosphate) was measured by monitoring the increase in absorbance at 412 nm at 37 °C. The amount of generated pnitrophenol was calculated from the molar absorptivity coefficient at pH 8, which was 17 000 mol/cm. Serum PON was expressed as U/L.[12]Phenylacetate was used as a substrate to measure ARYL by monitoring the increase of absorbance at 270 nm at 37 °C. Enzymatic activity was calculated from the molar absorptivity coef fi cient of the produced phenol, 1 310 mol/cm. One unit of ARYL was defined as 1 μmol phenol generated per minute under the above conditions, and expressed as U/L.[19]
Measurement of Cp and total sulfhydryl groups of serum samples
Cp enzymatic activity was measured according to Erel's method.[20]Using this method, ferrous ion was oxidized to ferric ion, via Cp ferroxidase activity. The results of this measurement were expressed as U/L. The -SH of the serum samples was assayed according to Ellman's method,[21]as modified by Hu et al.[22]The results of the assay were expressed as mmol/L.
Statistical analysis
COHb, PON, ARYL, Cp, and -SH were examined graphically using the Kolmogorov-Smirnov test. If the data of the test were normally distributed and independent, groups 1 and 2 were compared using the independentsample t test. If the data were normally distributed and dependent, Pillai's Trace test was performed to evaluate the differences at admission, at the 90thminute, and at the 180thminute of treatment. The relationship between variables was analyzed using Pearson's product-moment correlation test. The results were expressed in mean ± standard deviation. For statistical evaluation, we used SPSS for Windows Version 18.0 (SPSS Inc., Chicago, Illinois, USA). In all comparisons, P<0.05 was considered statistically signi fi cant.
RESULTS
In this study the average time of the patients between CO exposure and admission to the emergency department was 33.50±13.6 minutes. All the patients were conscious on arrival. Some of them were directly admitted to the emergency department of our hospital, and others were referred from other hospitals. In all patients, CO exposure was the result of the defective heating system. The clinical characteristics of groups 1 and 2 were comparable in terms of age, gender, body mass index, triglycerides, total cholesterol, HDL cholesterol and LDL cholesterol (Table 1). In both groups, age and gender were not correlated with the levels of PON, ARYL, Cp, and –SH. Nor correlation was observed between COHb levels and PON, ARYL, Cp and –SH levels in group 1. The difference between mean PON, ARYL, Cp, and –SH levels in men and women was not statistically signi fi cant in the two groups (P>0.05).
The comparison of COHb levels with PON, ARYL, Cp and –SH levels in group 1 was shown in Table 2. PON, ARYL, and -SH levels were lower in group 1 than in group 2 (P<0.001 for all), but Cp level was higher in group 1 than in group 2 (P<0.001) (Table 3). All the patients with COP were treated in the emergency department, and had a good recovery after discharge from the hospital.
DISCUSSION
Previously, COP was thought to be due to cellular hypoxia caused by replacing oxyhemoglobin with COHb, while producing a relative anemia.[23]COP is far more complex than expected and its pathophysiology is beyondthe formation of COHb. The pathophysiological effects of CO lead to problems in four areas: hemoglobin binding, direct cellular toxicity, heme-containing proteins-binding, and increases in oxidants, such as nitric oxide (NO).[23]These effects help to explain why COHb levels are not correlated with the severity of the clinical situation. If early and appropriate treatment is not provided, the combination of hypoxia/ischemia and direct cellular toxicity and the effects of oxidants may initiate a chain of events that result in severe disability or death.
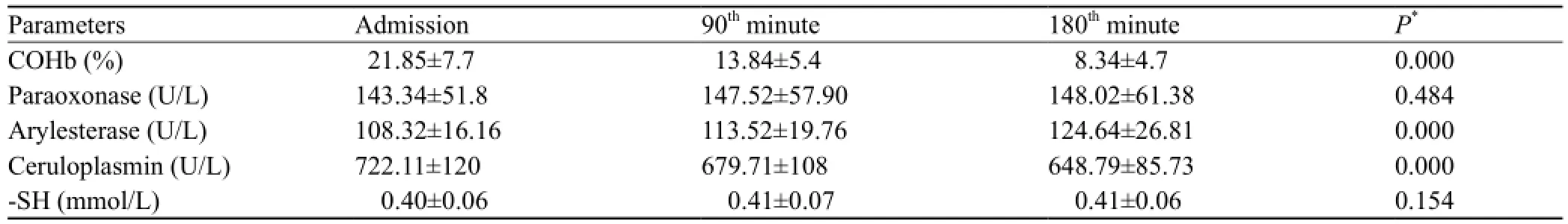
Table 2. Comparison of PON, ARYL and Cp activity, -SH levels (mean±SD)
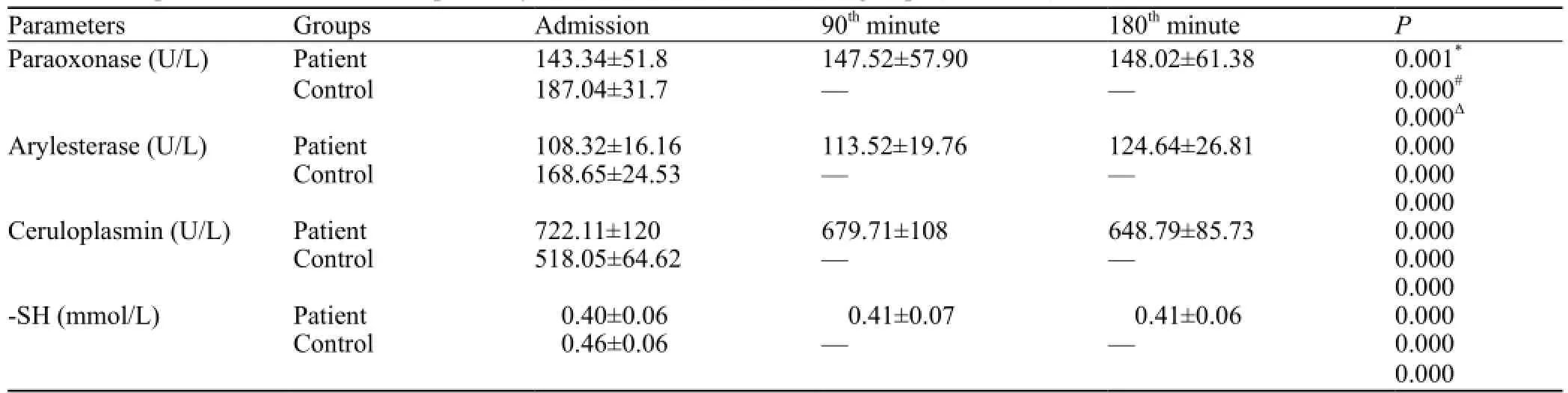
Table 3. Comparison of PON, ARYL, Cp activity, and -SH levels between both groups (mean±SD)
Oxidative stress can occur because of overproduction of oxidants, a decrease in antioxidant defenses or a combination of these factors. Organisms possess antioxidative mechanisms to overwhelm oxidants. The natural antioxidant system consisting of antioxidant enzymes and numerous antioxidant compounds protects functional and structural molecules against ROS, singlet oxygen, NO, superoxide anion, hydrogen peroxide, hydroxyl radicals, alkoxyl radicals, peroxyl radicals, and lipid peroxides-mediated cytotoxicity and tissue damage. In some conditions, oxidants increase and antioxidants decrease, and antioxidative mechanisms may be incapable of completely preventing oxidative damage. As a consequence, oxidative stress, which has been implicated in over 100 disorders, develops. Some of these disorders (such as diabetes mellitus, atherosclerosis, hypertension, cardiac dysrhythmia, and myocardial damage) are present in COP.[1,2,24]
There is no study on the relationship between COP and antioxidant sub parameters (PON, ARYL, Cp, and -SH). To our knowledge, ours is the first to examine PON, ARYL, Cp and -SH levels in patients with COP. Wang et al[25]demonstrated that CO-mediated delayed neuronal damage might be related to an increase of lipid peroxidation and a decrease of antioxidative status. The time-dependent changes of lipid peroxidation and antioxidative status in serum, cerebral cortex, and hippocampus are at least in part involved in the toxic effects of neuronal CO poisoning. Another study[26]demonstrated that COP increases the total oxidant status but does not change the total antioxidant status. Kavakli et al assessed total antioxidants, whereas we evaluated a limited number of antioxidants. In the present study, we found that PON and ARYL and -SH levels were signi fi cantly lower in group 1 than in group 2 (Table 3). The chemical mechanisms for this apparent decrease in antioxidant defenses have not been elucidated, and further analysis is required. Unlike other antioxidants, the mean Cp level was increased presumably because of a compensatory mechanism or an in fl ammatory response (Table 3). In our study we found that PON, ARYL, and -SH levels increased with time, whereas Cp level decreased (Table 2).
Oxidative stress induced by CO toxicity plays a significant role in various chronic conditions, such as cardiovascular diseases. Evidence of high intakes of antioxidants such as carotenoids, vitamin E, vitamin C, and selenium associated with a lower risk for many diseases has been shown in studies involving large groups of men and women.[27–29]The use of antioxidants also appears to be beneficial for tissue recovery after ischemic-reoxygenation injuries.[23]Therefore, antioxidant supplementation may lead to an increase in the antioxidant defense system and, thus, improvement in the clinical symptoms of COP.
In conclusion, we observed a significant decrease in PON, ARYL and -SH levels in patients with COP, indicating that these patients had a suppressed antioxidative status. However, these results are not suf fi cient to explain the relationship between antioxidants and COP. Further studies are required to clarify the possible mechanisms underlying the decreased andincreased levels of the factors.
Our study had several limitations. The patients with COP were not clinically very serious, so we could not evaluate the possible difference in antioxidant status with regard to serious cases. Moreover, only PON, ARYL, Cp, and -SH were examined in our study. An evaluation of more parameters may obtain better results and contribute to an increased understanding of pathophysiology of COP.
Funding:None.
Ethical approval:This study was approved by the Ethics Committee of Gaziantep University School of Medicine, Gaziantep, Turkey.
Conflicts of interest:The authors report no conflicts of interest regarding this study. The authors are responsible for the content of the paper.
Contributors:Zengin S proposed the study, analyzed the data and wrote the fi rst draft. All authors contributed to the design and interpretation of the study and to further drafts.
REFERENCES
1 Weaver LK. Clinical practice. Carbon monoxide poisoning. N Engl J Med 2009; 360: 1217–1725.
2 Kao LW, Nanagas KA. Carbon monoxide poisoning. Emerg Med Clin N Am 2004; 22: 985–1018.
3 Centers for Disease Control and Prevention. Carbon monoxide related deaths United States, 1999–2004, Morbidity and Mortality Weekly Report, December 21, 2007. MMWR Morb Mortal Wkly Rep 2007; 56: 1309–1312.
4 Al B, Yildirim C, Zengin S, Cavdar M, Togun I. The effect of chronic carbon-monoxide exposure on the peak expiratory flow values of grill-kebab chefs. Saudi Med J 2009; 30: 788–792.
5 Valko M, Leibfritz D, Moncol J, Cronin MT, Mazur M, Telser J. Free radicals and antioxidants in normal physiological functions and human disease. Int J Biochem Cell Biol 2007; 39: 44–84.
6 Karakaya A, İbiş S, Kural T, Köse SK, Karakaya AE. Serum paraoxonase activity and phenotype distribution in Turkish subjects with coronary heart disease and its relationship to serum lipids and lipoproteins. Chem Biol Interact 1999; 118: 193–200.
7 Aslan M, Kosecik M, Horoz M, Selek S, Celik H, Erel O. Assessment of paraoxonase and arylesterase activities in patients with iron de fi ciency anemia. Atherosclerosis 2007; 191: 397–402.
8 Canales A, Sanchez-Muniz FJ. Paraoxanase, something more than an enzyme? Med Clin (Barc) 2003; 121: 537–548.
9 Aviram M, Rosenblat M, Bisgaier CL, Newton RS, Primo-Parmo SL, La Du BNJ. Paraoxonase inhibits high density lipoprotein (HDL) oxidation and preserves its functions: A possible peroxidative role for paraoxonase. J Clin Invest 1998; 101: 1581–1590.
10 Watson AD, Berliner JA, Hama SY, La Du BN, Faull KF, Fogelman AM, et al. Protective effect of high density lipoprotein associated paraoxonase. Inhibition of the biological activity of minimally oxidized low-densitylipoprotein. J Clin Invest 1995; 96: 2882–2891.
11 Horoz M, Aslan M, Selek S, Koylu AO, Bolukbas C, Bolukbas FF, et al. PON1 status in haemodialysis patients and the impact of hepatitis C infection. Clin Biochem 2007; 40: 609–614.
12 Eckerson HW, Wyte CM, La Du BN. The human serum paraoxonase/arylesterase polymorphism. Am J Hum Genet 1983; 35: 1126–1138.
13 Baskol G, Baskol M, Kocer D. Oxidative stress and antioxidant defense in serum of patients with non-alcoholic steatohepatitis. Clin Biochem 2007; 40: 776–780.
14 Meritxell NM, Francisco JSM, Jose VSG, Elvira LO, Jose MSM. Determination of rat and mice arylesterase activity using serum mimetics. Enzyme and Microbial Technology 2008; 43: 252–256.
15 Messerschmıdt A, Huber R. The blue oxidases, ascorbate oxidase, laccase and ceruloplasmin modelling and structural relationships. Eur J Biochem 1990; 187: 341–352.
16 Keles MS, Taysi S, Sen N, Aksoy H, Akcay F. Effect of corticosteroid therapy on serum and CSF malondialdehyde and antioxidant proteins in multiple sclerosis. Can J Neurol Sci 2001; 28: 141–143.
17 Güngör N, Ozyürek M, Güçlü K, Cekiç SD, Apak R. Comparative evaluation of antioxidant capacities of thiol-based antioxidants measured by different in vitro methods. Talanta 2011; 83: 1650–1658.
18 Gur M, Aslan M, Yildiz A, Demirbag R, Yilmaz R, Selek S, et al. Paraoxonase and arylesterase activities in coronary artery disease. Eur J Clin Invest 2006; 36: 779–787.
19 Haagen L, Brock A. A new automated method for phenotyping arylesterase (E.C.3.1.1.2.) based upon inhibition of enzymatic hydrolysis of 4-nitrophenyl acetate by phenyl acetate. Eur J Clin Chem Clim Biochem 1992; 30: 391–395.
20 Erel O. Automated measurement of serum ferroxidase activity. Clin Chem 1998; 44: 2313–2319.
21 Ellman GL. Tissue sulfhydryl groups. Arch Biochem Biophys 1959; 82: 70–77.
22 Hu ML, Louie S, Cross CE, Motchnik P, Halliwell B. Antioxidant protection against hyochlorous acid in human plasma. J Lab Clin Med 1993; 121: 257–262.
23 Omaye ST. Metabolic modulation of carbon monoxide toxicity. Toxicology 2002; 180: 139–150.
24 Halliwell B. Free Radicals in Biology and Medicine. Oxford: Oxford Science Publications, 2000.
25 Wang P, Zeng T, Zhang CL, Gao XC, Liu Z, Xie KQ, et al. Lipid peroxidation was involved in the memory impairment of carbon monoxide-induced delayed neuron damage. Neurochem Res 2009; 34: 1293–1298.
26 Kavakli HS, Erel O, Delice O, Gormez G, Isikoglu S, Tanriverdi F. Oxidative stress increases in carbon monoxide poisoning patients. Hum Exp Toxicol 2011; 30: 160–164.
27 Fritz H, Kennedy D, Fergusson D, Fernandes R, Cooley K, Seely A, et al. Selenium and lung cancer: a systematic review and meta analysis. PLoS One 2011; 6: 1–10.
28 Bhupathiraju SN, Tucker KL. Coronary heart disease prevention: nutrients, foods, and dietary patterns. Clin Chime Acta 2011; 412: 1493–1514.
29 Talaulikar VS, Manyonda IT. Vitamin C as an antioxidant supplement in women's health: a myth in need of urgent burial. Eur J Obstet Gynecol Reprod Biol 2011; 157: 10–13.
Received October 22, 2013
Accepted after revision March 28, 2014
World J Emerg Med 2014;5(2):91–95
10.5847/ wjem.j.issn.1920–8642.2014.02.002



